Abstract
Objective: Stanford Neuromodulation Therapy (SNT) has demonstrated impressive efficacy for treatment-resistant depression (TRD), but its neurophysiological mechanisms remain poorly understood. This study investigated the neurophysiological correlates of SNT using transcranial magnetic stimulation with electroencephalography (TMS-EEG).
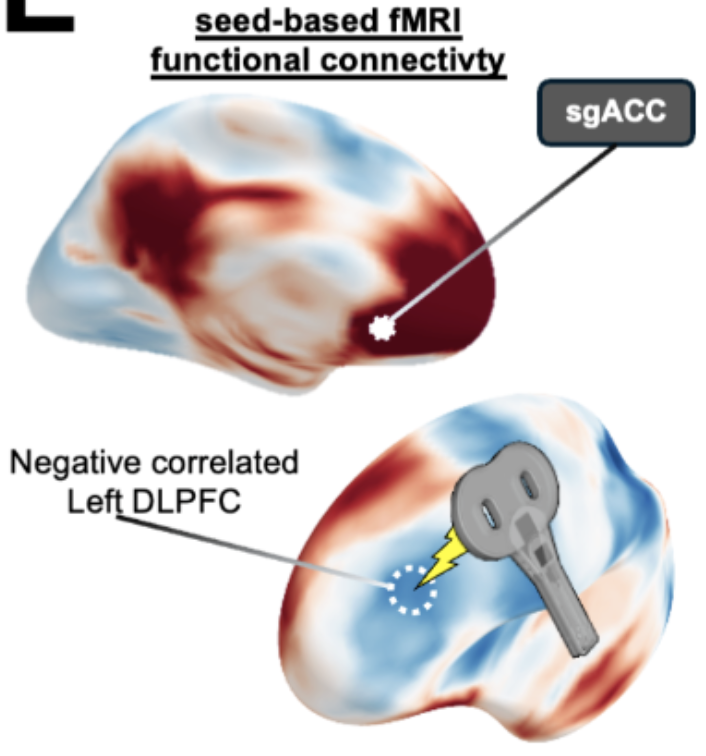
Method: A double-blind, randomized, sham-controlled clinical trial was conducted at Stanford University. Twenty-four participants with TRD (12 active, 12 sham) received TMS-EEG measurements at baseline, before/after each treatment day across the 5-day protocol, and at 1-month follow-up. The intervention consisted of active or sham SNT protocol (10 daily sessions of intermittent theta-burst stimulation to the left dorsolateral prefrontal cortex over 5 consecutive days). Main outcome measures included TMS-evoked potentials (a measure of cortical excitability) at both the L-DLPFC (treatment site) and vertex Cz (control site), and sgACC source-localized activation.
Results: Active SNT progressively reduced cortical excitability specifically at the treatment site, with significant reduction observed by day 3 (N45 [early negative component at ∼45ms]: -27.9%, p<0.01; P200 [late positive component at ∼200ms]: -39.8%, p<0.001), while no changes occurred at the control site (Cz) in either group (all p>0.05). This spatial specificity was confirmed by direct site comparisons showing significant reductions only at the L-DLPFC target (t(22)=-3.8, p<0.001). Additionally, active SNT significantly reduced sgACC responses to TMS across sessions (F(13,222)=4.93, p<0.001), with effects persisting at 1-month follow-up. Critically, baseline sgACC activation predicted treatment response (r=-0.67, p=0.023), with higher baseline activation associated with greater clinical improvement.
Conclusions: SNT produces progressive, site-specific modulation of TMS-evoked potentials (cortical excitability) at the targeted L-DLPFC and selective reduction in sgACC activation, with neurophysiological changes mirroring clinical improvement. The spatial specificity of effects confirms target-specific neuroplasticity. Baseline sgACC activity and changes in cortical excitability early in treatment represent promising biomarkers for personalizing TMS treatment for depression.